You are looking for an innovative dosage form for your plant-based or chemically synthesised active ingredient?
You already have an established cough syrup on the market? And you are planning to launch an additional attractive alternative – a more convenient, more aromatic one?
A contemporary dosage form that patients can take discreetly at work, while on the move or when in bed – is that what you would ultimately like to have?
If so, pharmaceutical pastilles from BOLDER Arzneimittel are ideal for your project.
Whether you have a specific marketing idea you wish to realise or would like to manufacture an existing pastille under pharmaceutical conditions:
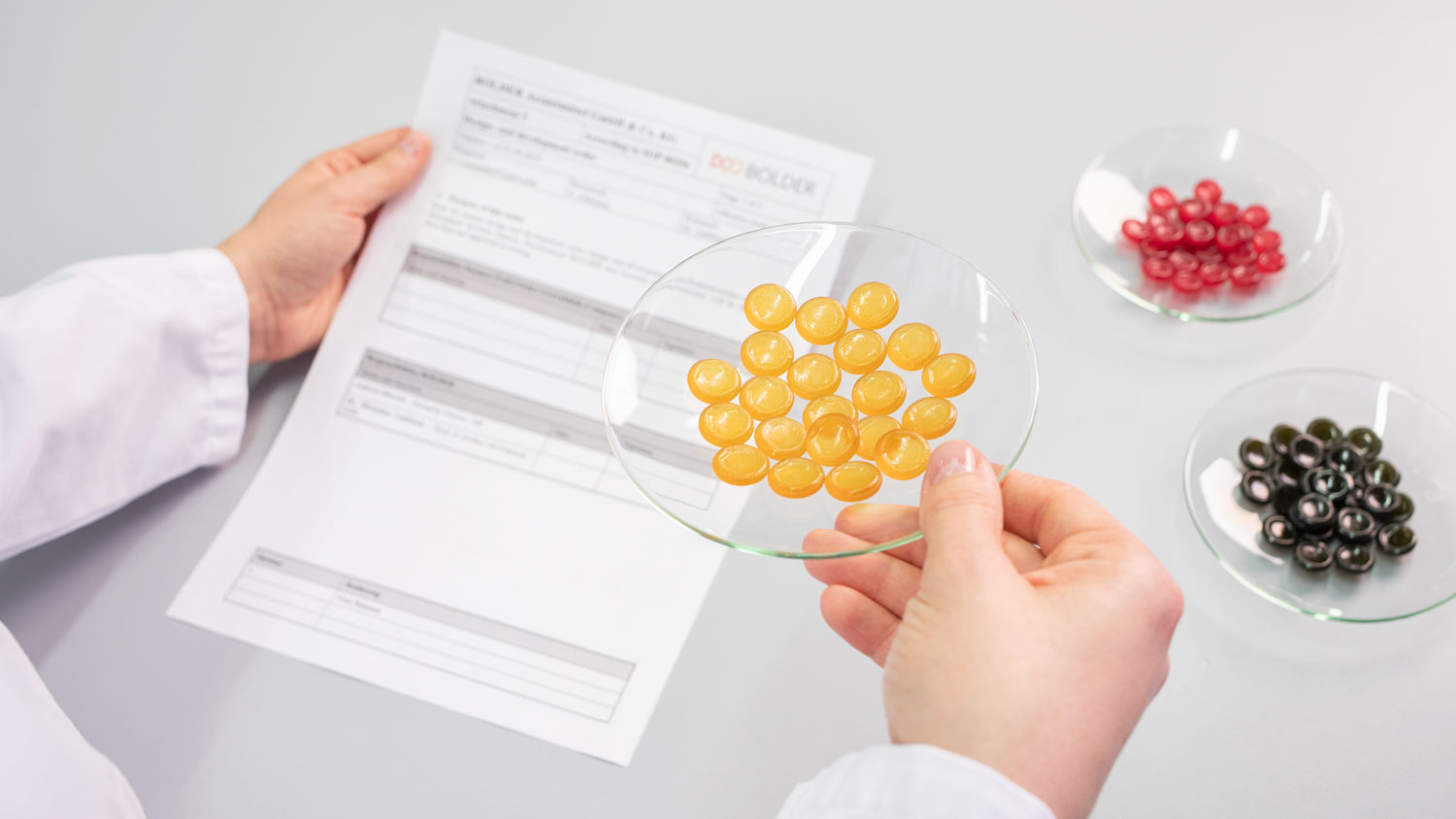
We custom develop and manufacture your product according to your wishes.
DEVELOPMENT PROJECT
We always treat your product as one-of-a-kind.
Pastilles produced by BOLDER Arzneimittel always meet the expectations of patients and the requirements of the market alike. We achieve this for you, amongst other things, by:
- making recommendations regarding the appropriate dosage, flavour and combination of active ingredients and excipients
- preparing form and flavour samples
- already factoring in international marketing considerations at the product design phase. These include such things as country-specific regulations, flavour preferences, climate zones and packaging.

Pharmaceutical-technological advantages of BOLDER pastilles
- BOLDER pastilles are solid solutions in single-dose form.
- They dissolve in the mouth and throat, slowly releasing their active ingredients.
- BOLDER pastilles have the same active ingredient homogeneity as true solutions.
ENCAPSULATION OF SENSITIVE SUBSTANCES
- BOLDER pastilles are based on acacia gum, which enables particularly sensitive substances to be incorporated by using the unique ActiSENSE® Technology.
- Many substances are very difficult or even impossible to process in other dosage forms.
- BOLDER Arzneimittel also manufactures pastilles using substances controlled under the German Narcotic Drugs Act.
Our development services at a glance:
- Advice on regulatory matters
(medicinal products, medical devices or food supplements) - Galenic development
- Scale-up, pilot batch production
- Method development and validation
- Stability programmes
- Preparation of approvals and registrations